ESA Technology Transfer Success Story - Dry electrodes to monitor vital signs: From astronauts in space to foetuses in utero • Aug 2022
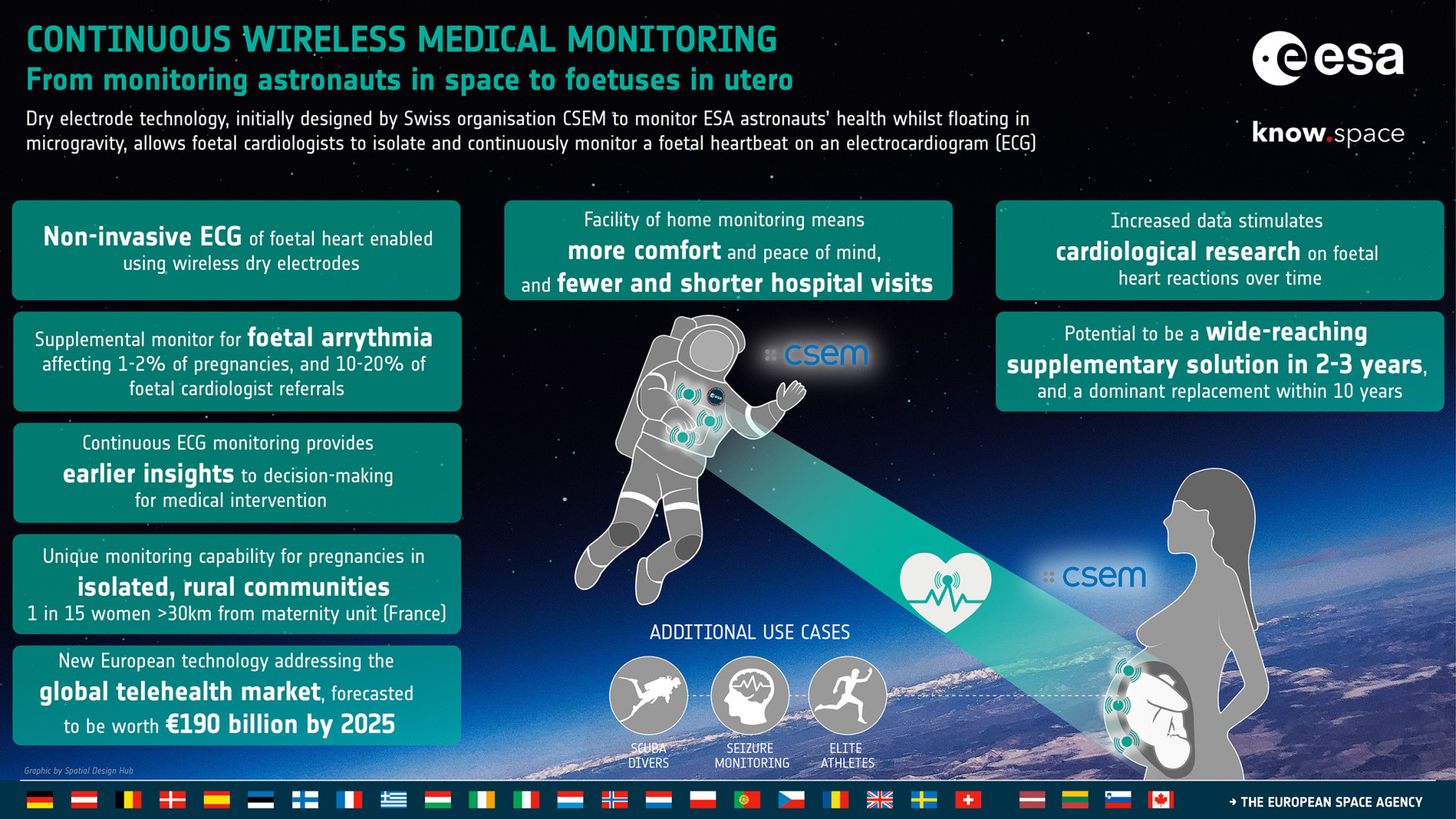
Dry electrode technology, initially designed by Swiss
organisation CSEM to monitor ESA astronauts’ health whilst floating in
microgravity (LTMS system), allows foetal cardiologists to isolate and
continuously monitor a foetal heartbeat on an electrocardiogram (ECG). CSEM saw
an opportunity to develop a technology (Electronic Foetal Monitoring Systemc - ELAINE)
using the dry electrodes that would provide a more comfortable wireless
solution, using ECG technology rather than CTG’s ultrasound solution.
Under ESA contract, CSEM developed the LTMS system, designed
to monitor astronauts’ vital signs using an easy to wear vest fitted with three
dry electrodes. This same technology is now being applied to foetal monitoring.
Before labour, it may be necessary to monitor the foetus’ heartbeat for any
irregularities, whereas during labour it is recommended to perform regular
monitoring of the foetal cardiac activity.
ELAINE may have the potential to supplement or replace
classical CTG machines in a hospital setting, but furthermore, this technology
could also pave the way for high-accuracy, at-home foetal monitoring. CSEM are
developing a portable device which could be used by women at home to monitor
their baby using a mobile phone app. Doctors could remotely monitor the foetus
and call mothers in to the hospital only when abnormalities are detected.
Offering a solution based on the ELAINE system will also position
them to offer a new product within the telehealth market, which is expected to
reach €190 billion globally by 2025. Through the collaboration of CSEM and its
partnering SME, there will also be potential revenue streams bringing economic
growth:
- For CSEM: a licensing scheme on the hardware and/or the algorithm.
- For the SME: Selling the device as a standalone hardware; subscription service for enhanced data analytics, usage of data acquired from patients in research studies with hospitals.
An increase in home monitoring by the patient means fewer
hospital visits, shorter stays at the hospital, and a decreased risk of
exposure of the patient to diseases such as COVID-19. A reliable home
monitoring solution where the mother can do a simple check of the foetal
heartbeat in order to alleviate some of their concerns could decrease the
number of times she would have to go in-person to the hospital. It also has the
potential to decrease the length of time a woman would have to remain at a
hospital for monitoring purposes. Using dry electrodes for ECG in foetal heart
monitoring has the potential to be a wide-reaching supplementary solution in
2-3 years, and a possible replacement method within 10 years. ELAINE can
provide additional supplementary monitoring solutions for pregnancies
displaying foetal arrythmia, which account for 1-2% of all pregnancies, and
10-20% of all referrals to foetal cardiologists.
The full case study report can be accessed in the restricted
area here
(please log in before).
The infographics can be accessed in the restricted area here (please log in before).
This initiative is led by ESA's Technology Transfer and Patent Management Unit (TTPO) in ESA's Directorate for Commercialisation, Industry & Procurement. The Unit is guiding start-ups, entrepreneurs and European businesses in developing spin-offs for ESA's space technologies. More recent successful transfers can be accessed at: Technology Transfer - Funded Projects. For more information, please contact patent@esa.int.
More articles of the category: Articles in news section
ESA Space Economy – Partnering with CERN to share best pract...
ESA Space Economy – Partnering with Eurostat and the Europea...
ESA Space Economy – Partnering with the OECD to develop inte...
ESA Space Economy – Understanding data on the space sector’s...
Seven Benefit Case Studies of ESA's Space Safety programme
ARTES benefits series – Pacis 3, Advancing Secure Government...
ARTES benefits series – Hybrid Connex, Digital Ambulance of...
ARTES benefits series – Eurialo, Global Real-Time Aircraft T...
ARTES benefits series – MRC-SAT, Real-Time Satellite Capacit...
ARTES benefits series – HummingSat, A Small Satellite with B...
Measuring the impacts of ESA programmes
Space Benefits for Earth - Preparing ESA Ministerial Council...
2nd Edition of the Workshop "Economics of Big Science" hoste...
First ever workshop on the “Economics of Big Science”
ESA Business Incubation Centres (ESA BICs) - Empowering spac...
ESA CM22 Economic Impact Report
ESA Science Programme: Empowering Europe’s Leadership in Cut...
ESA Benefit Case Studies – 100+ success stories of space for...
ESA Technology Market Assessments: understanding the foresee...
ESA pilots new framework to assess sustainability benefits a...
ESA launches new ITT to maximise sustainability benefits of...
Understanding the remarkable inspirational value of space ex...
Eurospace publishes the 2023 update of its facts & figures s...
Eurospace publishes the 2022 update of its facts & figures s...
Eurospace publishes the 2025 update of its facts & figures s...
Impact of ESA R&D (Discovery) on Europe’s innovation and res...
ESPI publishes its annual report on the private investment i...
ESA Business Applications and Space Solutions Success Storie...
ESA HQ welcomes the 2nd Edition of ECSECO Space Economy Days
NASA Economic Impact Report 2024
Impact of ESA R&D (TDE/GSTP) on Europe’s innovation and rese...
ESA FutureEO - Foundation for Europe's innovation and resear...
ESA Report on the Space Economy 2025
NASA’s Economic Impact Report 2022
ESA Discovery - Understanding the impact of ESA early R&D on...
ESA FutureEO - Foundation for Europe's innovation and resear...
U.S. BEA publishes updates on the U.S. space economy’s contr...
Eurospace publishes the 2024 update of its facts & figures s...
Copernicus Summer Series - Wildfire Management in Greece
Copernicus Summer Series - Irrigation Detection & Mapping in...
Copernicus Summer Series - Oil Spill in the Mediterranean
Copernicus Summer Series - Golf Course monitoring in Italy
ESA Technology Transfer Success Story - A new perspective: s...
When space technologies improve day-to-day life on Earth
Exports: an imperative for the European Space Industry? - A...
ECSECO, the European Centre for Space Economy and Commerce
ESA HQ welcomes the 1st Edition of the ECSECO Space Economy...
ESA ARTES Partnership Projects, providing the satcom industr...
European Human Space Transportation: Technological, Social a...
Upcoming event: ECSECO Space Economy Days
EUSPA publishes the 2nd Issue of its EO and GNSS Market Repo...
The OECD publishes the 2nd Edition of its flagship publicati...
Space Forum for Green Energy, an upcoming event from the ESA...
ESPI publishes its annual report on the private investment i...
FutureEO, critical enabler of EO benefits for the European e...
The OECD publishes the 2nd Edition of the Handbook on Measur...
Terrae Novae: from inspiring Europe’s generations to support...
ESA TIA ARTES programme’s continuous boost to the commercial...
Exploiting the remarkable potential of space technology tran...
Technology developments for ESA science missions empowering...
ESA Space Economy Team presents a paper on “Statistic and th...
European Centre for Space Economy and Commerce (ECSECO) pres...
ESPI Yearbook 2021 – Space Policies, Issues, and Trends of t...
ESA Science Core Technology Development Success Story - Broa...
ESA Science Core Technology Development Success Story - Game...
ESA Science Core Technology Development Success Story - Grou...
ESA Science Core Technology Development Success Story - Fost...
ESA Science Core Technology Development Success Story - Crit...
ESA Science Core Technology Development Success Story - Unri...
ESA Science Core Technology Development Success Story - Firs...
ESA Technology Transfer Success Story - From space debris to...
ESA Technology Transfer Success Story - Closing the loop: ho...
ESA Technology Transfer Success Story - Uncovering the secre...
ESA Technology Transfer Success Story - Landing zone assessm...
ESA Technology Transfer Success Story - Space at home: using...
Space-based Solar Power: Contributing to achieving Net Zero...
ESA Space Operations and Space Safety activities: supporting...
Europe decides to increase ESA’s budget by 17% compared to t...
European Centre for Space Economy and Commerce (ECSECO) conc...
OECD Policy Paper: How the War in Ukraine is affecting Spac...
Beyond Borders: Satellite Applications for Humanitarian Emer...
“Earth’s Orbits at Risk”, 2022 OECD report on the Economics...
ESA Technology Transfer Success Story - The missing layer: h...
ESA Technology Transfer Success Story - Powering a village f...
ESA Technology Transfer Success Story - No such thing as a w...
“International Space Station (ISS) Benefits for Humanity”; 2...
Valuing the benefits of ESA Aeolus missions to European deci...
European Centre for Space Economy and Commerce (ECSECO) offi...
ESPI Space Venture 2021 – Entrepreneurship and Investment in...
ESPI Space Venture 2022 – Investment in the European and Glo...
ECSECO open for membership registration on its official webs...
ESA Centre to develop Europe’s Space Economy and promote com...
The OECD Space Forum launches second phase of research oppor...
EARSC showcasing Copernicus uses for Environmental Complianc...
Creation of the European Centre for Space Economy and Commer...
ESA-Eurostat workshop on a European Space Economy Satellite...
EUSPA publishes EO and GNSS Market Report 2022
BEA’s “Estimating the United States Space Economy Using Inpu...
The European Commission publishes the 2021 Edition of its Be...
ESPI Yearbook 2020 – Monitoring the development of the Europ...
ESA Technology Transfer Success Story - Using space heritage...
EARSC workshop showcasing 24 Copernicus Sentinel value case...
The Canadian Space Agency publishes the 2021 & 2022 State of...
OECD’s examination of Space Technology Transfers and their C...
PwC’s ‘Lunar market assessment: market trends and challenges...
ESA Technology Transfer Success Story - Space-style control...
ESA Technology Transfer Success Story - Cities as Spaceships...
ESA joins the Universeh inaugural conference to address the...
ESA announces winners of the Global Space Markets Challenge
SPAC and the Space Industry
G20 Space Economy Leaders Meeting 2021
Top 12 companies selected in Global Space Markets Challenge
Global Space Markets Challenge: Longlists announced
Measuring how space creates jobs and prosperity on Earth
The Size & Health of the UK Space industry in 2019
Copernicus Sentinel data supporting the pulp and paper indus...
Entrepreneurship and private investment trends in the Europe...
Global Space Markets Challenge Competition
Metalysis–ESA Grand Challenge: team Malt wins first phase
The International, North American and European Statistical C...
OECD’s approach to space sustainability and the economics of...
China’s Space Sector: Commercialisation with Chinese Charact...
Space Architecture: Economic impacts, future developments an...
ESA_Lab@UCLan: Assessing the public value of ESA programmes
Call for participants: OECD's initiative on the value and su...
Big Science in the 21st Century – a new e-book published by...
Financing SMEs: options for SMEs and Midcaps in Europe
The socio-economic value of satellite Earth observations: hu...
Copernicus Sentinel Data supporting wine making in France
ESA_Lab@Kozminski: A new bird in the nest of ESA_Lab
ESA_Lab@PoliBa: De’ remi facemmo ali
OECD's analysis of the impacts of Covid-19 on the Space indu...
Post-crisis scenarios for the space industry
Resilience of the space sector to the Covid-19 crisis
Financing space: options for SMEs and midcaps in Europe
ESA and Metalysis Organised the First Grand Challenge Midter...
Watch again the GSEW Online
Non-space business? We want to hear from you
Join the Third Online Global Space Economic Workshop
Join the Second Online Global Space Economic Workshop
Join the First Online Global Space Economic Workshop
Watch again the GSEF 19
Interview with Eric Morel de Westgaver on Europe's space eco...
Global Space Economic Forum: Space Creates Value
Kick off of Metalysis – ESA Grand Challenge: the Race to Min...
ESA at the New Space Economy European Expoforum
Advancing the understanding and measurement of the societal...
ESA Space Economy Brochure
Space cybersecurity for smart cities
Space workshops to power urban innovation
Building and powering by disruptive innovation
Challenges of future urban settlements on the Moon and Mars
ESA at the New Space Economy European Expoforum
Two Teams Competing for a Half-million Prize
Creating value
ESA and Metalysis decide to suspend temporarily the Grand Ch...
Value created by ESA Telecommunication Partnership Projects
Value created by ESA's Future Earth Observation Pillar
Value created by ESA Science Programme
Value created by ESA's Ground Systems Engineering and Operat...
The socio-economic impact of space activities
Measuring the Space Economy
Value created by ESA's Clean Space Initiative
The Covid Crisis: for European SMEs, this could be a breakth...
Why it is important to keep investing in space during and af...
What is the Space Economy?
A closer look at the latest Earth Observation Services Indus...
ESA Global Space Economic Forum
Welcome to the Global Space Economic Workshop
The benefits of Copernicus’ Sentinel data to society, enviro...
ESA announces first Global Space Economic Forum
Global Space Economic Workshop
Discussing solutions at the Global Space Economic Workshop
Building cybersecurity at the Global Space Economic Workshop
Global Space Economic Workshop
Interview with Vincent Bastide on construction
A closer look at OECD’s methodology for assessing the scient...
Interview with Guglielmo Baeli on the oil and gas sector
Interview with Giulia Pastorella on cybersecurity
Last chance to join the competition
OECD’s analysis of the impacts of Covid-19 on the space indu...
ESA at Station F: looking for applications to the first Gran...
Compete in a lunar economy
The Moon Race: Pioneering Sustainable Lunar Exploration
Value created by ESA's Space Systems for Safety and Security...
Metalysis–ESA Grand Challenge launched
A closer look at the European Commission’s Guide to Cost-Ben...
Value created by ESA's planetary defence initiative and Hera...
A community of innovation at the Farnborough Airshow and ESA
Join us at Le Bourget to discuss space for commercial purpos...
Game changers for the ESA Grand Challenge
Setting the stage
Fuel the future by joining the Innovation Exchange
ESA Grand Challenge rewards solutions to complex problems
